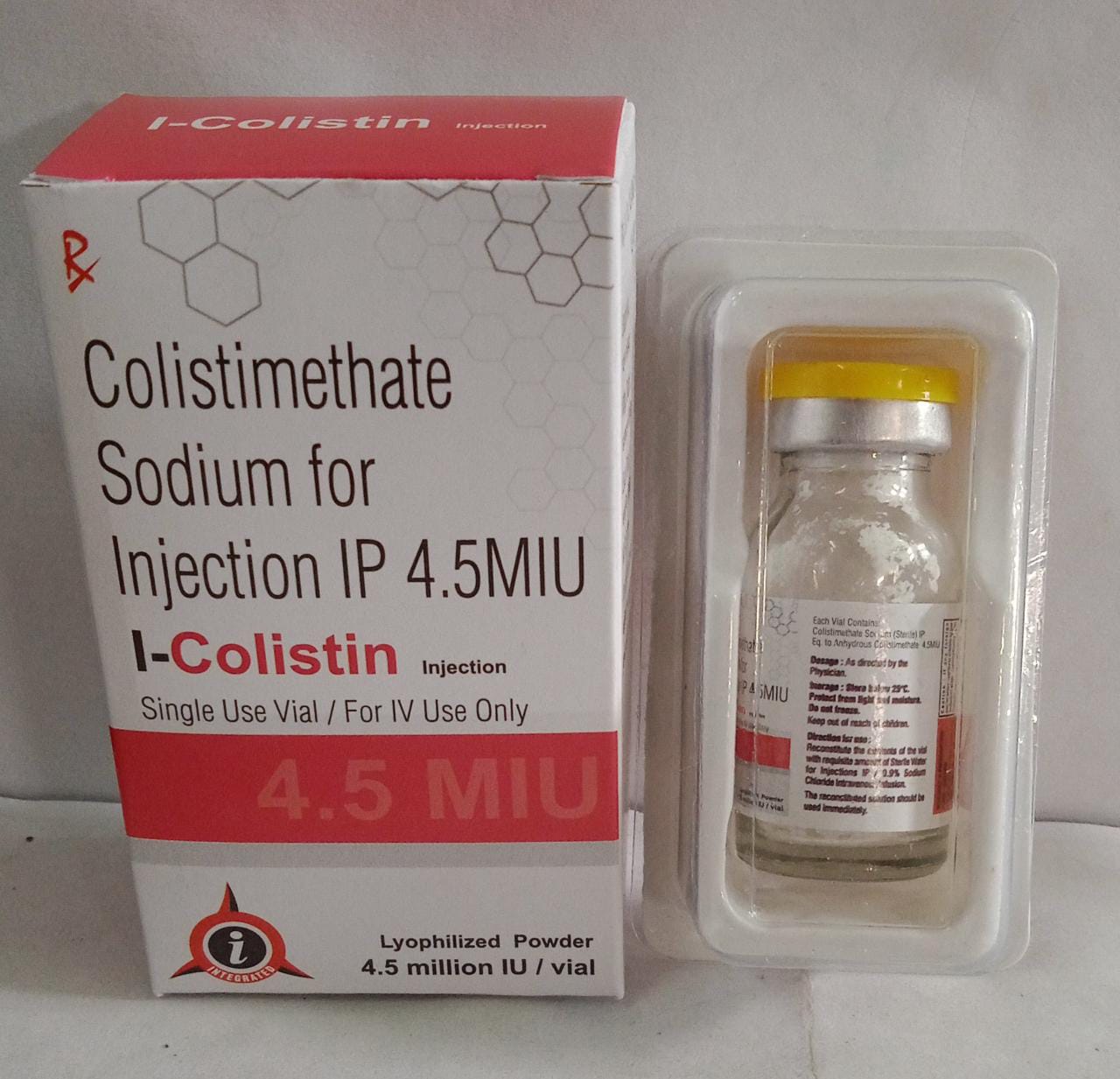
Colistimethate Injection (I Colistin 4.5 Miu)
Colistimethate Injection(I-Colistin 4.5 Miu)
Colistimethate injection is an antibiotic indicated for the treatment of infections caused by susceptible strains of certain gram-negative bacilli. It is particularly useful for targeting sensitive strains of Pseudomonas aeruginosa.
Colistimethate-4.5 Miu injection is a polymyxin antibiotic agent. Originally, colistimethate sodium was thought to be less toxic than polymyxin B; however, if the drugs are administered at comparable doses, their toxicities may be similar. Polymyxins are cationic polypeptides that disrupt the bacterial cell membrane through a detergent-like mechanism. With the development of less toxic agents, such as extended-spectrum penicillins and cephalosporins, parenteral polymyxin use was largely abandoned, except for the treatment of multidrug-resistant pulmonary infections in patients with cystic fibrosis. More recently, however, the emergence of multidrug-resistant gram-negative bacteria, such as Pseudomonas aeruginosa and Acinetobacter baumannii, and the lack of new antimicrobial agents have led to the revived use of polymyxins.
Mechanism of action
Colistimethate 4.5 Miu injection is a surface active agent which penetrates into and disrupts the bacterial cell membrane. Colistimethate is polycationic and has both hydrophobic and lipophilic moieties. It interacts with the bacterial cytoplasmic membrane, changing its permeability. This effect is bactericidal. There is also evidence that polymyxins enter the cell and precipitate cytoplasmic components, primarily ribosomes.
Click on the “Enquire” button to submit your requirement for Pcd pharma franchise or pharma third party manufacturing of this product.
Also For Higher Anitibiotics (Beta lactam) :- welcureremedies
Ascorbic Acid Injection (Welvit -C)
Tigecycline Injection(Tg-cillin 50)
Additional information
| Brand Name | I-COLISTIN 4.5 MIU |
|---|---|
| Packing | 10ML VIAL WITH TRAY |
Reviews
There are no reviews yet.